Dr Martin Dahl Discusses the Pathophysiology of AD and Benefits of ANB032
AnaptysBio's senior vice president of research, Martin Dahl, PhD, discusses therapeutic strategies and pathophysiological approaches to treating patients with atopic dermatitis (AD).
This content was produced independently by The American Journal of Managed Care® and is not endorsed by the American Academy of Dermatology.
ANB302 is a novel non-depleting antibody that has the potential to treat many systemic inflammatory diseases.
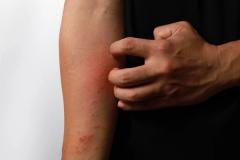
In a poster presented at the American Academy of Dermatology (AAD), ANB302 was associated with reduced inflammatory cytokines that drive the pathophysiology of atopic dermatitis (AD), according to Martin Dahl, PhD, senior vice president of research, AnaptysBio.
Transcript
What are the primary mechanisms underlying the pathophysiology of AD, including the role of immune dysregulation, skin barrier dysfunction, and genetic predisposition?
We find that in atopic dermatitis, it's a T-cell driven disease. So, when we look in the skin, we can see a lot of T cells that accumulate in the skin and they can be a heterogeneous population of T-cells, sometimes Th1 [T helper cell 1], sometimes Th2, sometimes Th17, sometimes Th22, or sometimes a mix of all of those types. [Emma Guttman, MD, PhD, professor, Icahn School of Medicine at Mount Sinai; director of the Occupational Dermatitis Clinic and director of the Laboratory for Inflammatory Skin Diseases] had a really great publication back in 2008, where she showed in histology, the presence of a high number of T cells. Not only are there a high number of T cells, [but] she also showed a really dense population of dendritic cells that are in the skin driving that inflammatory T-cell process.
Can you please provide an overview of the study presented at AAD on ANB032 for patients with AD?
At this meeting, we presented a poster on ANB032 around the dendritic cell biology in atopic dermatitis. In the past, we and others have shown that BTLA expression on T cells represents an immune checkpoint that can be added agonized to turn off the T-cell function. And what that does, it reduces T-cell proliferation, migration into tissue, and reduction of inflammatory cytokines across a broad panel of cytokines.
In this study, what we've done is we've moved on to the dendritic cell. We can see not only are there a lot of dendritic cells in the skin and patients, but we can take those dendritic cells, we can purify them, and when we stimulate them with an immune stimulant like LPS [lipopolysaccharide] to mature them, they upregulate a lot of BTLA on the surface, which allows us to engage that dendritic cell and try to turn it off through the agonistic mechanism.
What we did in this poster was we took immature dendritic cells, matured them overnight in LPS and looked at the upregulation of BTLA expression. Then we repeated the study by adding an ANB032 to agonize BTLA. In that process overnight, even in the presence of LPS, ANB032 agonized the dendritic cells and prevented them from maturing. So, we see a reduction in the number of cells that mature, a reduction in MHC [major histocompatibility complex] class 2 expression, which is needed for T-cell activation, and a reduction in the costimulatory molecules CD80, CD86, and CD40.
The combination of that effect should reduce T-cell priming and reduce the activity and the ability of dendritic cells to activate T cells downstream. We then took those dendritic cells that were cultured with ANB032 overnight and they were in the immature state. We washed off all the drug and we took those DCs [dendritic cells] and put them in a culture with naive T cells. And that's a mixed lymphocyte reaction, where typically the dendritic cell will activate the T-cells and drive an inflammatory process.
If we pretreat those DCs with ANB032, what we saw was the induction of a large number of regulatory T cells that express FOXP3 and CD25. And those regulatory T cells contributed to reducing the inflammatory cytokines from other T cells in that culture. So, we saw reductions in IL-17, reduction of interferon gamma, and reductions of IL-5; all common T-cell cytokines that drive the pathology of atopic dermatitis.
Newsletter
Stay ahead of policy, cost, and value—subscribe to AJMC for expert insights at the intersection of clinical care and health economics.