Dr Lawrence Eichenfield Hopeful for New Pediatric AD Treatment Option
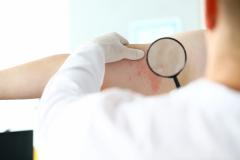
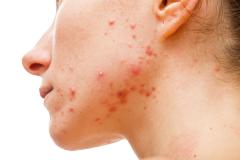
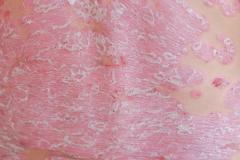
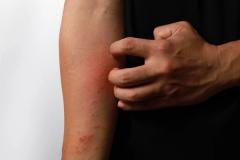
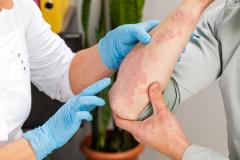
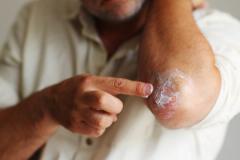
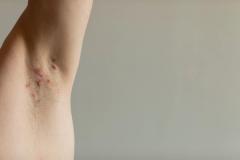
Lawrence Eichenfield, MD, chief of pediatric and adolescent dermatology at Rady Children's Hospital in San Diego, shared the implications of the findings from INTEGUMENT-PED, the phase 3 study assessing the efficacy and safety of once-daily roflumilast cream 0.05% in pediatric patients with atopic dermatitis (AD), for future clinical practice.
This content was produced independently by The American Journal of Managed Care® (AJMC®) and is not endorsed by the American Academy of Dermatology.
At the
In an interview with AJMC, Eichenfield said that roflumilast cream 0.05% had positive results in pediatric patients with AD in terms of efficacy and safety. He noted that he is looking forward to roflumilast cream being "hopefully approved" and "potentially having this in our armamentarium for treating young children."
Transcript
How do the results of the INTEGUMENT-PED study contribute to our understanding of AD treatment options for young children? What implications might they have for clinical practice and future research?
First, to go through some of the basics, the study hit the sort of standard outcome measures that we look for. What percentage of patients made it to clear or almost cleared? About 25% of those were within a 4-week time period with once-a-day application with a big delta, or almost 15% delta, between the vehicle.
It was very interesting to me that a lot of the population that got that clear, or almost clear, were already clear or almost clear by 2 weeks, and there was almost 80 something percent of those because it was 21% of the patients made clear or almost clear at week 2.
The secondary outcome measures being clear or almost clear at EASI [Eczema Area and Severity Index] 75, and the percentage of patients who had at least a 4-part drop in itch as compared to their baseline was consistent with a nice separation from the vehicle cream throughout the course of the study.
When we looked very early, the improvement in the itch with roflumilast was seen as early as 24 hours after the first application. So, it was a very rapid separation of itch scores from the vehicle.
Overall, I think having this move forward and hopefully be approved as a new nonsteroidal, which is very well formulated, can be helpful in our care of patients with atopic dermatitis. We show in this case that it helped to improve eczema in 2- to 5-year-olds with early clinical responses and very good tolerance levels. So, we look forward to potentially having this in our armamentarium for treating young children.
Newsletter
Stay ahead of policy, cost, and value—subscribe to AJMC for expert insights at the intersection of clinical care and health economics.