
The elimination of the program addressing LGBTQ+ individuals on the national suicide hotline makes reaching specialized help harder, says Hannah Wesolowski of the National Alliance on Mental Illness.

The elimination of the program addressing LGBTQ+ individuals on the national suicide hotline makes reaching specialized help harder, says Hannah Wesolowski of the National Alliance on Mental Illness.

Rising grocery prices are forcing most Americans to shift toward ultraprocessed foods, leading to increased health risks and exacerbating existing disparities, particularly among underserved communities.

Lorna Warwick, CEO of the Lymphoma Coalition, highlights findings underscoring the vital role of clinician communication in managing adverse effects and supporting patient confidence in lymphoma and chronic lymphocytic leukemia (CLL) care.

Up to 257 million Americans could benefit from these prior authorization reforms that could have cross-market implications on health care plans administered through commercial insurers, Medicare Advantage, and Medicaid.

From widening racial health disparities and abortion care restrictions to coverage losses, leadership gaps in addressing social needs, and worsening workforce shortages, the urgent need to improve health equity is underscored in the US.
Circulating tumor DNA (ctDNA) testing changes occur nearly every year, making consistent updates to National Comprehensive Cancer Network (NCCN) guidelines important for clinicians to make decisions on the use of these methods of testing, says Eric Lander, MD.

The FDA approved datopotamab deruxtecan-dlnk (Datroway; Daiichi Sankyo) for the treatment of locally advanced or metastatic EGFR-mutated non–small cell lung cancer (NSCLC) after receipt of EGFR-directed therapy and platinum-based chemotherapy.

This research highlights the key factors, like leadership buy-in, mandatory protocols, and electronic health record workflows, that influence the effective collection of data on sexual orientation and gender identity in outpatient oncology clinics to improve patient-centered care.

Combining physician education with patient previsit activation increases deprescribing of diabetes medications, according to one study.

Widespread noncompliance with federal cost-sharing rules exists, particularly impacting Medicare beneficiaries and patient-preferred prep options, study finds.

Patients can benefit both financially and clinically by enrolling in ongoing clinical trials, said Eric Lander, MD.

The FDA has approved Monjuvi (tafasitamab) for relapsed follicular lymphoma, offering hope with improved progression-free survival in combination therapy.

Lorundrostat shows promise in reducing blood pressure and proteinuria in patients with hypertension and chronic kidney disease (CKD), highlighting its dual-action benefits.

The recent FDA approval of lenacapavir is encouraging in its promise of long-term HIV prevention but might not be available for the vast majority of people in the US.

It's a critical time to spotlight the dire health disparities affecting Black Americans, from premature deaths to maternal care, and the urgent need for systemic reforms.

There was no association observed between lower income and increased fibrosis among New York City residents with metabolic dysfunction-associated steatohepatitis (MASH).

The approval of lenacapavir, a form of pre-exposure prophylaxis (PrEP), marks significant progress in preventing HIV, making it vital for the treatment to be available and accessible to those most vulnerable, explains Colleen Kelley, MD, MPH, Rollins School of Public Health at Emory University.

For patients with obesity, clinicians should move beyond a "try and fail" approach to lifestyle interventions and prioritize weight management medication when it can deliver crucial cardiovascular benefits.

Dupilumab was approved by the FDA to treat bullous pemphigoid, that demonstrated its efficacy in achieving sustained disease remission and significantly improving symptoms like itch and disease severity in adults.

Coverage of our peer-reviewed research and news reporting in the health care and mainstream press.
Multi-hit TP53 mutations significantly worsened survival in patients with myeloproliferative neoplasms (MPNs) and acute myeloid leukemia (AML), revealing critical prognostic insights.
Venetoclax demonstrated impressive efficacy in octogenarians with CLL, although treatment management posed some challenges.
Patients who see a cardiologist at least once a year are about 24% less likely to die in the following year.

Telitacicept is a dual-targeting agent that attaches to and blocks the effects of 2 key signaling proteins, B-lymphocyte stimulator and a proliferation-inducing ligand, to treat the B-cell–mediated autoimmune disease, generalized myasthenia gravis.
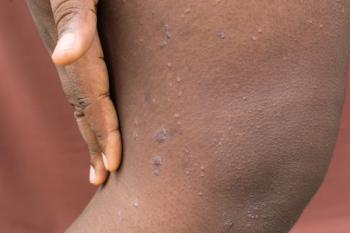
Nemolizumab significantly and safely improved itch and skin lesions for up to 2 years in patients with moderate to severe prurigo nodularis.

The approval of lenacapavir for use as pre-exposure prophylaxis is a significant step in reducing the incidence of HIV across the globe, including in areas where the PURPOSE trials were conducted.

Early trials found that both garadacimab and sebetralstat were safe when used in patients living with hereditary angioedema, which speaks to the promise of the FDA-approved garadacimab.

Making clinical trials easier to find for clinicians and making protocols for entering clinical trials more lenient can help to improve access to clinical trials on a national and local level, explains Eric Lander, MD, of Minnesota Oncology.

Surbhi Sidana, MD, MBBS, director of the Myeloma Disease Focused Group at Stanford University, provides a reintroduction to CARTITUDE-4 and insight on how this phase 3 investigation builds on previous findings of CAR T vs standard-of-care findings in relapsed/refractory multiple myeloma (RRMM)

Median overall survival was 5 months longer in patients with small cell lung cancer (SCLC) who received tarlatamab instead of chemotherapy after progression following platinum-based chemotherapy.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
