
The newly approved linvoseltamab (Lynozyfic; Regeneron) has a manageable safety profile, along with a patient-friendly dosing schedule that includes intravenous administration, step-up dosing, and de-escalation to monthly maintenance.

The newly approved linvoseltamab (Lynozyfic; Regeneron) has a manageable safety profile, along with a patient-friendly dosing schedule that includes intravenous administration, step-up dosing, and de-escalation to monthly maintenance.

Many FDA accelerated approvals continue to rely heavily on surrogate endpoints, raising ongoing uncertainty about the true clinical benefits of these therapies.

Finerenone gained FDA approval for heart failure treatment, showcasing significant benefits in reducing cardiovascular events, based on FINEARTS-HF trial results.

The treatment marks the only targeted oral treatment in patients with non–small cell lung cancer with epidermal growth factor receptor exon 20 insertion mutations.

For the second time in under 3 weeks, the FDA has approved a treatment for the inherited genetic disorder characterized by recurrent episodes of severe swelling.

This approval comes almost a year after the drug received a complete response letter due to third-party manufacturing issues.

FDA issues a complete response letter for oxylanthanum carbonate, a treatment for hyperphosphatemia in patients with chronic kidney disease (CKD) on dialysis.

Belimumab is now the first and only subcutaneous biologic therapy approved for at-home administration in pediatric lupus nephritis.

The FDA approved datopotamab deruxtecan-dlnk (Datroway; Daiichi Sankyo) for the treatment of locally advanced or metastatic EGFR-mutated non–small cell lung cancer (NSCLC) after receipt of EGFR-directed therapy and platinum-based chemotherapy.

In this interview with TRUST-I and TRUST-II trial investigator, Jorge J. Nieva, MD, USC Keck School of Medicine, he walks through the design of the trials, the results that supported the FDA approval, and taletrectinib’s potential to redefine first-line therapy.

The FDA has approved Monjuvi (tafasitamab) for relapsed follicular lymphoma, offering hope with improved progression-free survival in combination therapy.

The approval of lenacapavir, a form of pre-exposure prophylaxis (PrEP), marks significant progress in preventing HIV, making it vital for the treatment to be available and accessible to those most vulnerable, explains Colleen Kelley, MD, MPH, Rollins School of Public Health at Emory University.

Dupilumab was approved by the FDA to treat bullous pemphigoid, that demonstrated its efficacy in achieving sustained disease remission and significantly improving symptoms like itch and disease severity in adults.

Early trials found that both garadacimab and sebetralstat were safe when used in patients living with hereditary angioedema, which speaks to the promise of the FDA-approved garadacimab.

The recent approval of garadacimab can help to treat patients with hereditary angioedema (HAE) along with sebetralstat, which is awaiting FDA approval, explains Timothy Craig, DO, Penn State Health.

With the approval of the FDA, garadacimab can help patients with hereditary angioedema (HAE) prevent attacks by shutting down the contact pathway.

Garadacimab-gxii is the first and only treatment that targets factor XIIa (FXIIa) for the prophylactic prevention of hereditary angioedema (HAE) attacks in patients 12 years and older.

The FDA has granted approval to pembrolizumab for the treatment of adult patients with resectable, locally advanced HNSCC whose tumors express PD-L1 with a CPS of ≥1.

The FDA approved mitomycin intravesical solution (Zusduri) for patients with recurrent low-grade intermediate risk non–muscle-invasive bladder cancer (NMIBC).

The tablet formulation of zanubrutinib (Brukinsa; BeOne) is now approved for all 5 indications across several hematological cancers.

This next-generation ROS1 tyrosine kinase inhibitor previously received breakthrough therapy and orphan drug designations for the same patient population, as well as additional non–small cell lung cancer (NSCLC) indications.

The CDC’s Advisory Committee on Immunization Practices was expected to meet later in June to issue recommendations for use.

With the FDA approval of retifanlimab (Zynyz; Incyte) as the first and only first-line treatment for advanced anal cancer, researchers are now focusing on resistance mechanisms and future therapies, according to Sheela Rao, MBBS, MD, FRCP, of The Royal Marsden Hospital.

In May, the FDA granted significant drug approvals across various therapeutic areas, marking notable progress in expanding treatment options for patients.

Darolutamide (Nubeqa) is granted FDA approval for metastatic castration-sensitive prostate cancer, enhancing survival and quality of life for patients.

Gerard Criner, MD, FACP, FACCP, MATINEE investigator, highlights trial results that showed reduced exacerbations and delayed disease progression in patients with eosinophilic chronic obstructive pulmonary disease (COPD).
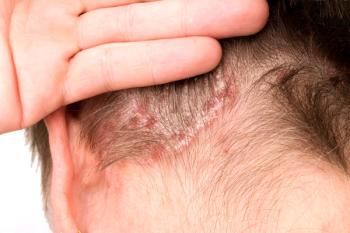
Once-daily roflumilast provides skin clearance and rapid itch relief with no limitation on duration of use.

FDA approves mepolizumab (Nucala; GSK) as the first monthly biologic for chronic obstructive pulmonary disease (COPD), significantly reducing exacerbations in patients with an eosinophilic phenotype.

The FDA previously approved Jivi in 2018 to treat adults aged 12 years and older with hemophilia A.

The FDA approved retifanlimab (Zynyz; Incyte) as the first and only first-line treatment for advanced anal cancer, supported by data from the POD1UM-303/InterAACT2 and POD1UM-202 trials.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
