Standard of Care and Guidelines for Psoriasis
Experts provide the standard of care and a discussion of the guidelines for therapy.
Transcript
Peter L. Salgo, MD: Let’s go on to the standard of care, which is a very nice segue here, I think. Somebody comes in with psoriasis and receives their initial diagnosis; what is the standard of care right off the bat?
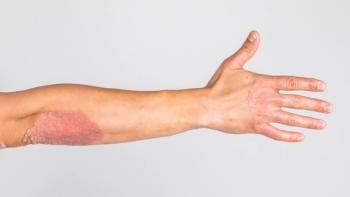
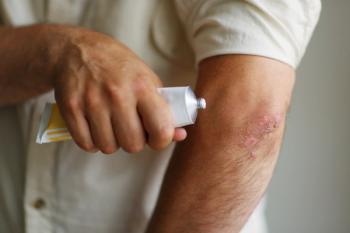
Steven Feldman, MD, PhD: I’d say you’d have to classify the psoriasis as either relatively limited or very extensive. If it’s relatively limited—it’s just on the elbows and knees—then the standard of care would be some kind of topical therapy. You could do localized ultraviolet light treatments, which would be an alternative, but basically, you’re likely to start with a cream, seeing if that would work. If people have so much body coverage that creams are unlikely to work, or if they have psoriatic arthritis, the cream’s not going to fix that, so some kind of total body approach is in order. For a long time, we thought phototherapy would be the way to go for these people, and if that’s reasonable and feasible, that may be a perfectly good first-line option. Before biologics were introduced, methotrexate was the next thing to do for people. But because the biologics are more effective and safer than methotrexate, I would say the biologics are first-line. And because they’re so much easier, I think a lot of patients might prefer to just take an injection once in a while than receiving light treatment.
Peter L. Salgo, MD: If I hear you correctly, we’re moving into the 21st century, wherein we now understand the biologic mechanism of damage, if you will, and that we have drugs to treat it as opposed to just phototherapy, for example—not that phototherapy doesn’t work.
Joel Gelfand, MD, MSCE, FAAD: Right. It needs to be patient-centered. And so, really, what we’re doing is explaining to the patient what their disease is about, what their treatment options are. And for those who have more extensive disease or are more resistant to topical therapies, we talk about ultraviolet light approaches, oral medication approaches, and injectable biologic approaches.
Peter L. Salgo, MD: One of the wonderful things I get to do on these panels is to ask questions of specialists that I would love to ask, and I’m going to ask it now, because I’ve wondered about this forever. A lot of ultraviolet light we know is carcinogenic, so what’s the skin cancer rate in people getting a lot of light box therapies?
Joel Gelfand, MD, MSCE, FAAD: This is a great question, you would think it would be substantially elevated. But for our more modern uses of ultraviolet light with something called narrowband phototherapy, a specific wavelength of light, which is very effective for psoriasis, there have only been a few studies that look at this, and none of them have shown higher rates of skin cancer.
Peter L. Salgo, MD: Really?
Joel Gelfand, MD, MSCE, FAAD: You want to look at thousands and thousands of patients for many, many years. What clinicians will often say or what our experience tends to be is that, if there’s higher rates of skin cancer from it, it’s modest enough that most patients don’t seem to mind it. But it’s certainly a concern.
Steven Feldman, MD, PhD: I think that’s very well said. There must be some increased risk.
Peter L. Salgo, MD: Sure.
Steven Feldman, MD, PhD: But studies from the Mayo Clinic, where they gave people tar and light—the old kind of light—they followed people for decades; I don’t think they saw any detectable increased risk. And I think the reason for this is, most of the skin cancers happen where the sun hits you on a regular basis—the head and shoulders. Your head gets so much light over time. The psoriasis on the body, when we’re treating it with light, those areas have not had a lot of light. So you could probably give those areas a lot of light before they’ll ever start developing skin cancer.
Peter L. Salgo, MD: Because it’s a total accumulated dose is what you’re saying.
Steven Feldman, MD, PhD: Yes.
Peter L. Salgo, MD: There are guidelines, right? The AAD [American Academy of Dermatology] guidelines, the AAD-NPF [National Psoriasis Foundation] guidelines for the treatment of this. Have they been updated recently? What do they say at this point?
Steven Feldman, MD, PhD: The guidelines can’t be up to date because you put together this panel and you give them evidence and you spend some years doing this, and then they come out with guidelines that are 3 years out of date from the day they do it. And my impression of the guidelines that have been produced are guidelines of the things that are appropriate, and then you let the dermatologists use their judgment.
Joel Gelfand, MD, MSCE, FAAD: That’s right, because each patient is so individual in terms of their comorbidity profile, their preferences, their willingness to try different therapies or what’s available to them. That’s really what dictates decision making with the patients. I’m part of the AAD-NPF guidelines. They are just rolling out now starting in 2018, 2019, and so on. And there’s so much progress going on that I find them hopefully useful for providers, as well as for payers.
There are a lot of circumstances that aren’t traditionally addressed like what therapies might be useful on the palms and soles or, for example, not every patient does well with standard doses of, say, ustekinumab. And there are good level A data showing that you could increase the dose of ustekinumab in patients and have better outcomes with the same safety profile.
Peter Dehnel, MD: Can I put out there that there may be an obvious question? There is, believe it or not, a little difference in the range of cost with some of these products.
Peter L. Salgo, MD: You think? I bet tar is cheap.
Peter Dehnel, MD: As somebody who may be in a utilization review capacity, a patient with significant psoriasis comes in, says, “Hey, doc, I saw on the TV this great new product for treatment of psoriasis. I can then go to the locker room and I’m fine, people are not going to see my skin being affected. I can go to the swimming pool again.” I’m trying to get my arms around that because there are a number of products, some of which are subcutaneous, some of which now are oral. They don’t have to sit in an infusion center for 1 to 2 hours every other month.
Newsletter
Stay ahead of policy, cost, and value—subscribe to AJMC for expert insights at the intersection of clinical care and health economics.