Clinical
Latest News
Latest Videos

CME Content
More News
The GATHER1 and GATHER2 studies are highlighted.

Medical experts recap the DERBY and OAKS studies.

Hypercholesterolemia impact on cardiovascular outcomes is explored by a key opinion leader.

Because biologics are used to treat both conditions, researchers hypothesized they may be helpful to prevent psoriatic arthritis (PsA).

James F. Howard, Jr, MD, professor of neurology at the University of North Carolina at Chapel Hill, explains the antibody subtypes associated with this rare neuromuscular disease.

Chimeric antigen receptor (CAR) T-cell therapies have shown efficacy and safety for relapsed or refractory diffuse large B-cell lymphoma (DLBCL), but barriers to referral may obstruct patient access to them.

A secondary analysis of the phase 3 TOURMALINE-MM4 trial found that ixazomib as postinduction maintenance therapy for multiple myeloma produced a progression-free survival (PFS) benefit vs a placebo across age and frailty subgroups.

Perfluorohexyloctane (NOV03) was able to reduce the signs and symptoms of dry eye disease (DED) when compared with hypotonic saline control and was well tolerated.
The SPOTLIGHT study results presented at the 2023 ASCO GI Cancers Symposium are explored by Dr Marshall.

Each trial extension has added new and more data and a better understanding of how ruxolitinib cream treats vitiligo in the long term, explained John Harris, MD, PhD, FAAD, director of the Vitiligo Clinic and Research Center at UMass Chan Medical School.

A multidisciplinary approach and symptom management are both vital components of home care to provide continuity of care after cardiac surgery because they decrease symptoms and readmissions to the hospital and increase self-efficacy.

The findings suggest recombinant ADAMTS-13 may be a useful adjunct therapy to plasma exchange among patients who have thrombotic thrombocytopenic purpura (iTTP).
Many gene therapies promise life-changing effects, but without long-term data it remains to be seen how long the benefits last.

A new report from AHIP indicates that biosimilars have the potential to garner savings of $180 billion over 5 years, but there needs to be a review of the approval process for interchangeability to encourage more approvals.

While there are benefits of gene therapy, some patients will continue to need anti–vascular endothelial growth factor (VEGF) therapy to treat wet age-related macular degeneration (AMD), said Charles C. Wykoff, MD, PhD, of Retina Consultants of Texas and the Blanton Eye Institute at Houston Methodist Hospital.

While the future is bright with potential for personalized medicine to treat atopic dermatitis, current treatment is still more like trial and error, said Emma Guttman-Yassky, MD, PhD, FAAD, of the Icahn School of Medicine at Mount Sinai.

A study of patients with acute myeloid leukemia (AML) in first remission found that the presence of certain variants indicative of measurable residual disease ahead of allogeneic hematopoietic cell transplant (HCT) was associated with relapse and worse survival.

The patient’s thrombotic thrombocytopenic purpura (TTP)–like symptoms were caused by deficiencies of vitamins B1 and B12.

Dr Michos offers strategies to successfully reduce LDL levels in patients with cardiovascular disease.

GA treatment options in various steps in FDA approval process are discussed by a panel of medical experts.

Maria Lopes, MD, MS, highlights the utilization of health care resources to optimize GA treatment impact.
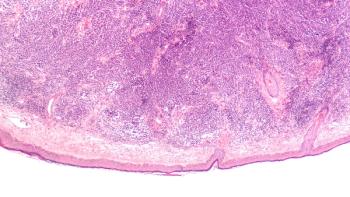
The patient sought care for a mass in her right buttock, but had no tonsillar symptoms.
Patients who underwent sublobar resection and those who underwent more invasive lobectomy for early-stage non–small cell lung cancer (NSCLC) showed similar overall and disease-free survival outcomes in a recent study.
Treatment with relugolix and concomitant therapies for prostate cancer showed similar safety and efficacy to relugolix alone in a recent study.
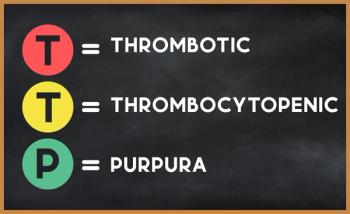
The impaired reactivity may play a role in organ injury, the authors suggested, among patients who have immune-mediated thrombotic thrombocytopenic purpura (iTTP).