Immuno-Oncology
Latest News
Latest Videos
CME Content
More News
Treatment resistance is a significant challenge in immunotherapy, but it is not fully understood. A recent review summarized 5 tumor microenvironment traits that may inhibit therapy effectiveness and potential strategies to improve therapy responses.

Miruna Sasu, PhD, MBA, president and CEO of COTA Healthcare, discusses the medicines discovery process and how real-world data can be used to evaluate patient and tumor response to treatment regimens.
The study of constructed αROR1‐CAR T cells showed an increase of PD-L1 expression when exposed to elevated pressure, driving resistance to CAR T cell-mediated cytotoxicity. However, the findings also suggest that adding PD-1/PD-L1 inhibitors to CAR T-cell therapy could help address this.

The researchers highlighted a need for the detection of more relevant markers and guidance on how to treat adverse events (AEs), the types and severity of which vary based on disease type.

So far, the wonders of chimeric antigen receptor (CAR) T-cell therapy have only been seen in blood cancers. Investigators at Moffitt Cancer Center report findings that show a potential target in solid tumors.

With further research, a selection of combination therapies could increase patient response in various cancer types.

Pacritinib is now included as a recommended treatment for myeloproliferative neoplasms with specificity for JAK2 and IRAK1 in the latest National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines.

Accenture’s 2021 report, “The Future Is Now: How to Drive Precision Oncology Adoption," adds to the chorus calling for greater sharing of data and improved standardization, so that academic centers and community practices alike can continually improve data sets used worldwide.

Camille Hertzka, vice president, head of oncology, US Medical, AstraZeneca, clarifies testing for the HRR mutation in metastatic castrate-resistant prostate cancer (mCRPC) and the predictive importance of radiographic progression-free survival (rPFS) for overall survival in this setting.

Therapy resistance remains a challenge with immune checkpoint inhibitors, and a new study proposes targeting ligand-dependent corepressor (LCOR) in addition to known immune targets to improve outcomes.
Immune checkpoint inhibitors have changed the treatment landscape of certain cancer types, and a recent study aims to provide new insight on why treatment response varies so much from patient to patient.

The expanded use of axi-cel, sold as Yescarta, while not unexpected, nonetheless represents uncharted territory in cancer care.

Camille Hertzka, vice president, head of oncology, US Medical, AstraZeneca, discusses why so much excitement has been generated for the use of olaparib (Lynparza) in the first line for patients with metastatic castrate-resistant prostate cancer.
Despite progress in chimeric antigen receptor (CAR) T-cell therapy, further research is needed to fully understand and overcome CAR T-cell exhaustion and improve outcomes.
Mantle cell lymphoma is a difficult cancer type with high relapse rates, but novel targeted approaches such as CAR T-cell therapy hold promise for more successful response rates in the future.

Despite current challenges in targeting pancreatic cancer with CAR T-cells, novel targets and strategies hold promise in this difficult disease setting.

Women are known to experience more adverse events with chemotherapy, but a recent study found an increased risk across therapy types, especially immunotherapy.

Although chimeric antigen receptor (CAR) T-cell treatments have shown efficacy in certain cancers, targeting multiple antigens and overcoming production limitations may advance the field and make this therapy more effective and accessible.

Camille Hertzka, vice president, head of oncology, US Medical, AstraZeneca, discusses why it is important to test for HRR gene mutation status and appropriateness of olaparib use in patients with metastatic castrate-resistant prostate cancer (mCRPC).
FDA approval is based on CARTITUDE-1, a phase 1b/2 trial in which investigators reported that cilta-cel produced an objective response rate (ORR) of 98% and a stringent complete response rate of 78%.
Research has shown that adoptive immunotherapy using natural killer cells may be beneficial in leukemia treatment. The follow-up to a recent study found that the dose of alloreactive natural killer cells matters for treatment response.
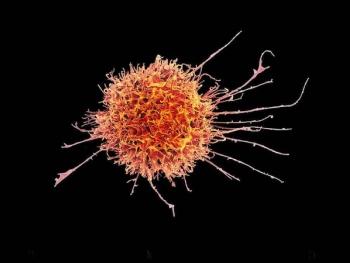
A recent review examined natural killer–based therapies, a type of immunotherapy that holds promise for treating leukemia with less toxicity than currently available therapies.

Lessons from the early days of chimeric antigen receptor (CAR) T-cell therapy remain fresh as Kite Pharma prepares for the FDA to act on its supplemental biologics license application for use of axi-cel as second-line therapy in relapsed or refractory large B-cell lymphoma. A target action date is set for April 1, 2022.

A statement from NCCN’s Advisory Committee on COVID-19 Vaccination and Pre-exposure Prophylaxis said that the panel “endorses vaccination for all eligible persons based on FDA-approved indications or emergency use authorization” and emphasized the need for everyone to be fully vaccinated—including third doses.

While the number of trials for cancer immunotherapies grew significantly, combination therapies being studied increased while monotherapies decreased.