
Melissa Andel, principal at CommonHealth Solutions, previewed her session on policy making and pricing reform at the upcoming Academy of Managed Care Pharmacy's annual meeting.

Skylar is an associate editor for The American Journal of Managed Care® (AJMC®) and The Center for Biosimilars®, and joined AJMC® in 2020. She is responsible for covering all aspects of the ever-changing global biosimilar industry and produces content that is accessible and informative for all health care stakeholders.
She has a BA in journalism and media studies from Rutgers University. You can connect with Skylar on LinkedIn.

Melissa Andel, principal at CommonHealth Solutions, previewed her session on policy making and pricing reform at the upcoming Academy of Managed Care Pharmacy's annual meeting.

Investigators confirmed an association between fatty liver disease related to metabolic dysfunction (MAFLD) and chronic kidney disease (CKD), and stressed a greater focus on treating MAFLD is needed to help manage risks.

A new frailty-based outcome prediction model for multiple myeloma (MM) was found to be an easy-to-use tool in clinical practice and produced valid results based on a large real world cohort of patients with a new MM diagnosis, according to a recent study.

During the recent Association of Community Cancer Centers’ 2022 Annual Meeting & Cancer Center Business Summit, panelists suggested that the laser focus on rebates by pharmacy benefit managers (PBMs) may be hindering uptake of biosimilars, thereby keeping some off of formulary lists.
Risk stratification is a difficult process for multiple myeloma (MM), and authors concluded that their multi-tiered model may provide a greater risk assessment benefit than standard stratification systems.

Genome sequencing of tumors from children and adolescents with recurrent or refractory cancer led to over 100 patients receiving targeted therapies.

Finerenone (Kerendia) was found to reduce risk of heart failure and improve other cardiovascular outcomes in patients with chronic kidney disease (CKD) and type 2 diabetes (T2D) regardless of heart failure history.

As the United States awaits market introduction for adalimumab biosimilars in 2023, 2022 is going to be the year of expanding access to biosimilars, according to Julie M. Reed, the new executive director of the Biosimilars Forum.

Researchers found that low levels of vitamin D and the prevalence of peripheral neuropathy were common in patients with multiple myeloma, suggesting that addressing the former could help prevent the latter.

Decorin, a highly conserved, class 1 small leucine-rich repeat proteoglycan expressed in the extracellular matrix, may have a protective effect in a subset of patients with multiple myeloma, investigators concluded.
Investigators concluded that early palliative care may be needed for more patients with multiple myeloma (MM) as pain can manifest in different ways and severity levels depending on disease state, suggesting that a more individualized approach is critical for pain management.

Releuko, a filgrastim biosimilar developed by Kashiv Biosciences and Amneal Pharmaceuticals, becomes the third filgrastim biosimilar to be approved by the FDA.

Robert Gabbay, MD, PhD, of the American Diabetes Association (ADA), talks about the impact that finerenone could have on patients with kidney disease and diabetes.

Thermo Fisher Scientific will begin accepting submissions for grant proposals focusing on molecular profiling in cancer spaces, where applicants could receive up to $200,000 in funding.

Biocon Biologics announced that it will be acquiring Viatris’ biosimilars portfolio for close to $3 billion.
A study showed that patients with multiple myeloma (MM) deal with physical, social, and emotional pain and that physicians did not fully comprehend the extent to which patients experienced pain, suggesting improved communication between parties is warranted.

Health care providers need to foster a greater push for physical activity as a part of survivorship care for patients with multiple myeloma (MM), investigators concluded after finding that only 25% of patients were active.

Julie M. Reed, the new executive director of the Biosimilars Forum, offers her opinion on how interchangeability will impact US biosimilar uptake now that the FDA has given the designation to 2 biosimilars.

Robert Gabbay, MD, PhD, of the American Diabetes Association (ADA), breaks down the reasoning behind the organization's decision to add Bayer's Kerendia (finerenon) as a recommended drug for kidney disease prevention.

The COVID-19 pandemic has resulted in significant disparities in mortality and made ever-present systemic issues worse for historically underserved communities, signaling that greater efforts to address social determinants of health (SDOHs) are needed now more than ever.

Access to digital health tools to improve patient outcomes and quality of life are lacking among patients with multiple myeloma (MM) who are elderly, at risk of relapse, or live in rural communities, suggesting more tools and guidelines are needed for these populations.

Robert Gabbay, MD, PhD, of the American Diabetes Association, explains why a section on chronic kidney disease management was added to the ADA's 2022 standard of care guidelines.

Two cancer-related micro-RNAs could serve as prognostic biomarkers for multiple myeloma bone disease as lower levels were associated with lower overall survival rates, investigators concluded.

Foundation Medicine’s circulating tumor DNA (ctDNA) detection and monitoring assay, FoundationOne Tracker, was granted a Breakthrough Device Designation from the FDA, streamlining the approval and review processes to give patients and providers earlier access to the device.

Although the COVID-19 pandemic has led to mass slowdowns in drug approvals and biosimilar launches, 2021 signaled a stronger focus on biosimilars going forward, with new regulatory bills and a crackdown on anticompetitive practices.

The organization announced a partnership with over 1000 nephrology providers to help implement CMS’ new Kidney Care Choices models beginning this year.

Robert A. Gabbay, MD, PhD, chief scientific and medical officer at the American Diabetes Association, shares his thoughts on long COVID-19 among those with type 2 diabetes and the rise of new-onset cases of type 1 diabetes throughout the pandemic.

Adding risk factors specific to patients with chronic kidney disease (CKD) to risk prediction models for cardiovascular disease (CVD) could improve risk prediction capabilities for a population of patients often subject to invasive diagnostic procedures, investigators concluded.
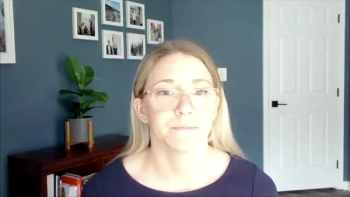
Megan Olsen, MPH, principal at Avalere, explains how payers are attempting to get around cost concerns associated with expensive cell and gene therapies.

Researchers concluded that incident corneal adverse events in patients with multiple myeloma (MM) carry a low clinical and economic burden compared with total all-cause costs and MM-related per-patient-per-month costs.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
