
FDA has granted sonrotoclax priority review for relapsed mantle cell lymphoma, showcasing promising trial results.

FDA has granted sonrotoclax priority review for relapsed mantle cell lymphoma, showcasing promising trial results.
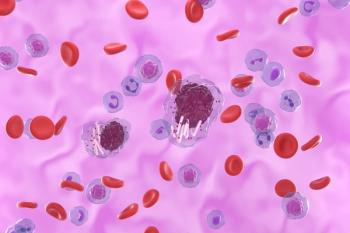
Treatment with liso-cel induced long-term complete remission rates and high undetectable minimal residual disease rates in patients with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma following progression on Bruton tyrosine kinase inhibition and venetoclax.

Tovorafenib generated durable drug holiday responses in pediatric patients with BRAF-altered relapsed/refractory low-grade glioma.

The FDA approved the FoundationOne Liquid CDx to identify patients with metastatic non–small cell lung cancer (mNSCLC) with MET exon 14 skipping alterations who may be eligible for tepotinib (Tepmetko; EMD Serono).

The European Medicines Agency's (EMA’s) Committee for Medicinal Products for Human Use (CHMP) has recommended the marketing authorization of mirvetuximab soravtansine (Elahere; AbbVie) for folate receptor alpha–positive (FRα+), platinum-resistant epithelial ovarian cancer.


Several experts that we spoke with for ASCO 2024 share their thoughts on why the American Society of Clinical Oncology annual meeting is so important to the field of oncology.

This new analysis of nivolumab presented at ASCO 2024 shows the immune checkpoint inhibitor did not convery benefit when added to a regimen of neoadjuvant carboplatin, paclitaxel, and radiation.

The National Comprehensive Cancer Network (NCCN) recommends ropeginterferon alfa-2b as first-line cytoreductive therapy for polycythemia vera.

Published: June 4th 2024 | Updated:

Published: June 10th 2024 | Updated:
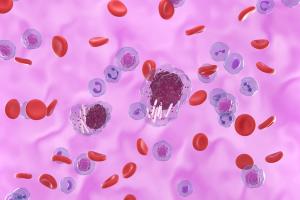
Published: March 7th 2025 | Updated:

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
